Spatial relationship between intestinal cell types and metabolism: We investigate the intricate spatial interactions and relationships between different intestinal cell types and their impact on metabolic processes. We explore the mechanisms that enable epithelial stem cells to acquire the nutrients necessary for their survival and to maintain their specific cell identities or fates.
How nutrient transporters act as 'metabolic gates' influencing stem cells and malignant transformation: We delve into the role of nutrient transporters as pivotal regulators, acting as 'metabolic gates' that influence the behaviour of stem cells and their potential transformation into cancer cells. We investigate the feasibility of targeting these nutrient transporters as potential therapeutic strategies for the treatment of cancer.
Research Interests
Cell biology revolves around a fundamental concept: cells require nutrients to survive, to support proper tissue and overall organismal function. In multicellular organisms, cells of different types tightly control this nutrient exchange from their environment depending on their metabolic requirements. While intracellular metabolic and nutrient sensing pathways are widely studied, our understanding of how cells control their nutrient and metabolite uptake in the first place remains largely unexplored. This essential nutrient acquisition process is primarily orchestrated by the intestine, serving as the central hub for nutrient sensing and absorption within the body.
In our laboratory, we study the general principle and mechanisms employed by intestinal cells to secure the vital nutrients and metabolic resources essential for preserving their stem cell fate and function. And, how these mechanisms can be exploited or disrupted during injury or damage and in metabolic disorders and cancer. How the plasticity of metabolism facilitate tumour formation? What contributions does this metabolic plasticity play during metastasis or therapies? Which challenges and opportunities arise from this?
We have previously discovered how cellular metabolism changes during colon cancer initiation and progression (Najumudeen et al., 2021) and combining multi-omics approaches can identify new targets for colon cancer (Vande Voorde et al., 2023). We have shown that oncogene dosage of cancer cells alters their signalling programme and thereby affect cancer metastasis (Najumudeen et al., 2024; Fey et al., 2025). For our studies, we use genetically engineered mouse and organoid models, which allow the functional perturbation of candidate genes in vivo to study metabolic interactions within the healthy and disease setting. We are developing novel tools to perform single-cell spatial metabolomics, perform CRISPR screens, in vivo gene editing and a whole plethora of biochemical and genetic approaches to uncover novel mechanistic insights.
Ongoing Projects
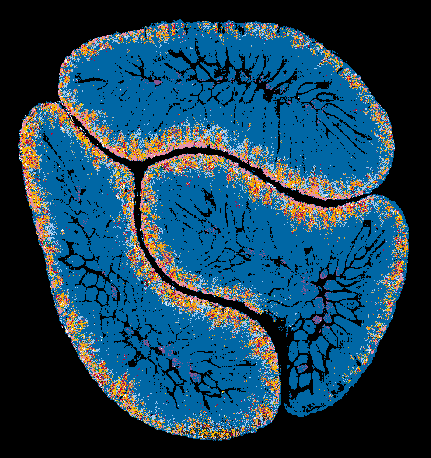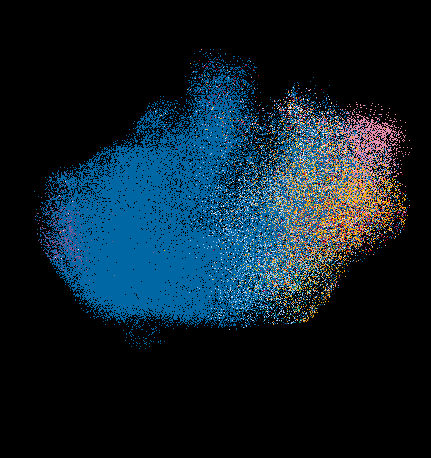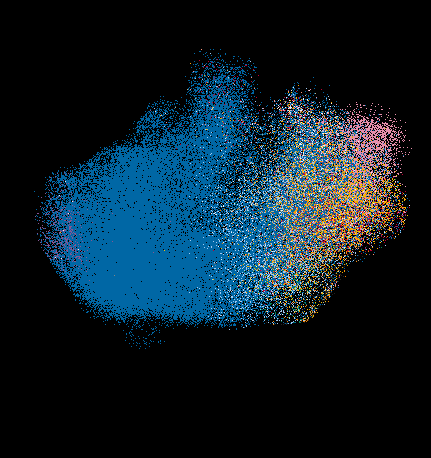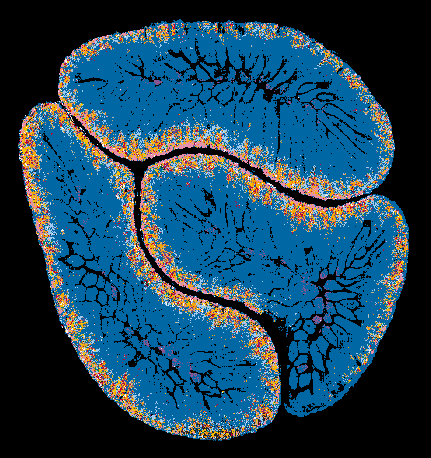

A spatial metabolite ion composite image of mouse small intestine showing metabolic compartmentalization. Image acquired with DESI mass spectrometry imaging in negative ion mode.
© Najumudeen lab.
Functional and metabolic heterogeneity of KRAS mutations, amplifications and gene dosage: Using preclinical model systems we investigate the non-equivalent tumorigenic potential and modes of action of clinically relevant KRAS mutations during tumour progression and therapy resistance in different cancer types using clinically relevant KRAS inhibitors and combinations.
Addressing these questions is integral to gaining key insights into how environmental factors, such as nutrition, exert control over health outcomes. Our research is supported by state-of-the-art laboratory facilities and a team of dedicated scientists. We collaborate with researchers from around the world to leverage the latest technologies and advance our understanding of the spatial organization of metabolism in cells and these metabolic dependencies in cancer cells.
Funding
None of our work would be possible without the support we receive from our generous funders past and present. Currently, our work receives support from:
Other partners
CRUK Cancer Grand Challanges
CRUK RADNET